Abstract
Background: The molecular landscape of acute myeloid Leukemia (AML) is heterogeneous, yet only a few mutations are targetable. Mutations in the isocitrate dehydrogenase genes (IDH1, IDH2) are heterozygous gain of function point mutations with an approximate frequency of 20%. FDA approved IDH2 inhibitor enasidenib in 2017 and IDH1 inhibitor ivosidenib in 2018 for adults with relapsed/refractory (R/R) IDH2 and IDH1MT AML. In May 2022, FDA approved ivosidenib combined with azacitidine for adults aged 75 years or older with newly diagnosed AML who cannot undergo intensive induction chemotherapy. Since IDH inhibitors (IDHi) have been in clinical use within and outside clinical trials for the last few years, we sought to retrospectively analyze our experience and clinical outcomes of patients with IDH mutations who were treated with/without IDHi at our center, especially there is some recent evidence of better response to venetoclax based therapy for patients with IDH mutations.
Methods: We included all patients diagnosed with a myeloid neoplasm with IDH1 or IDH2 or both mutations as identified by targeted Next Generation Sequencing using the commercially available Illumina TruSight Myeloid Panel and were treated between October 2014 and September 2021. Baseline demographic and clinical characteristics were noted. The Chi-square test was used to study various parameters as described in the results, and Kaplan-Meier curves were used for survival analyses.
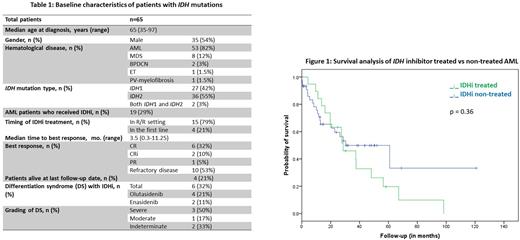
Results: Among 65 patients with myeloid neoplasms, 53 (82%) had AML, 8 (12%) had Myelodysplastic syndrome, 2 (3%) had Blastic Plasmacytoid Dendritic Cell Neoplasm (BPDCN), 1 (1.5%) had essential thrombocythemia, and 1 (1.5%) had myelofibrosis secondary to polycythemia vera. Of the 65, 54% (35/65) were male, and the median age at disease diagnosis was 65 years [range, 35-97]. 42% (27/65) in the cohort had IDH1 mutation, 55% (36/65) had IDH2 mutation, and 3% (2/65) had both. Only 29% (19/65) of patients in the cohort received IDHi therapy, and all had AML. 12 (63%) were female. 15 (79%) patients received IDHi therapy in the R/R setting and 4 (21%) in the first line. The median age of patients receiving IDHi was 66 years [range, 44-80]. Of the 19 patients who received IDHi, 6 (32%) achieved complete remission (CR), 2 (10%) achieved complete remission with incomplete count recovery (CRi), 1 (5%) had a partial response (PR), and 10 (53%) had refractory disease. The median time to best response was 3.5 months [range, 0.3-11.25]. IDHi-induced differentiation syndrome (DS) was reported in 6 patients (32%); 4 (21%) who developed DS received Olutasidenib, and 2 (11%) received Enasidenib. Three patients (50%) developed severe DS, two of which died from it. One had moderate DS, and 2 had indeterminate DS. All patients with DS were treated with medication discontinuation and intravenous dexamethasone. Median overall survival for the IDHi group was 28.6 months (95% CI 16.0-41.3) and 30.4 months (95% CI 5.9-54.9) for the non-IDHi group (p = 0.363).
Conclusion: Only a minority of patients with IDHMT AML had received IDHi-based therapy, which is attributed to patients diagnosed and treated before the FDA approval. Surprisingly, the median survival for patients who didn't receive IDHi therapy seemed not different, underscoring the other therapies' effects, including the influence of the venetoclax-based regimen. More extensive randomized studies are needed to compare the efficacy of IDHi to other therapies in treating IDHMT AML.
Disclosures
Balasubramanian:Kura Oncology: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal